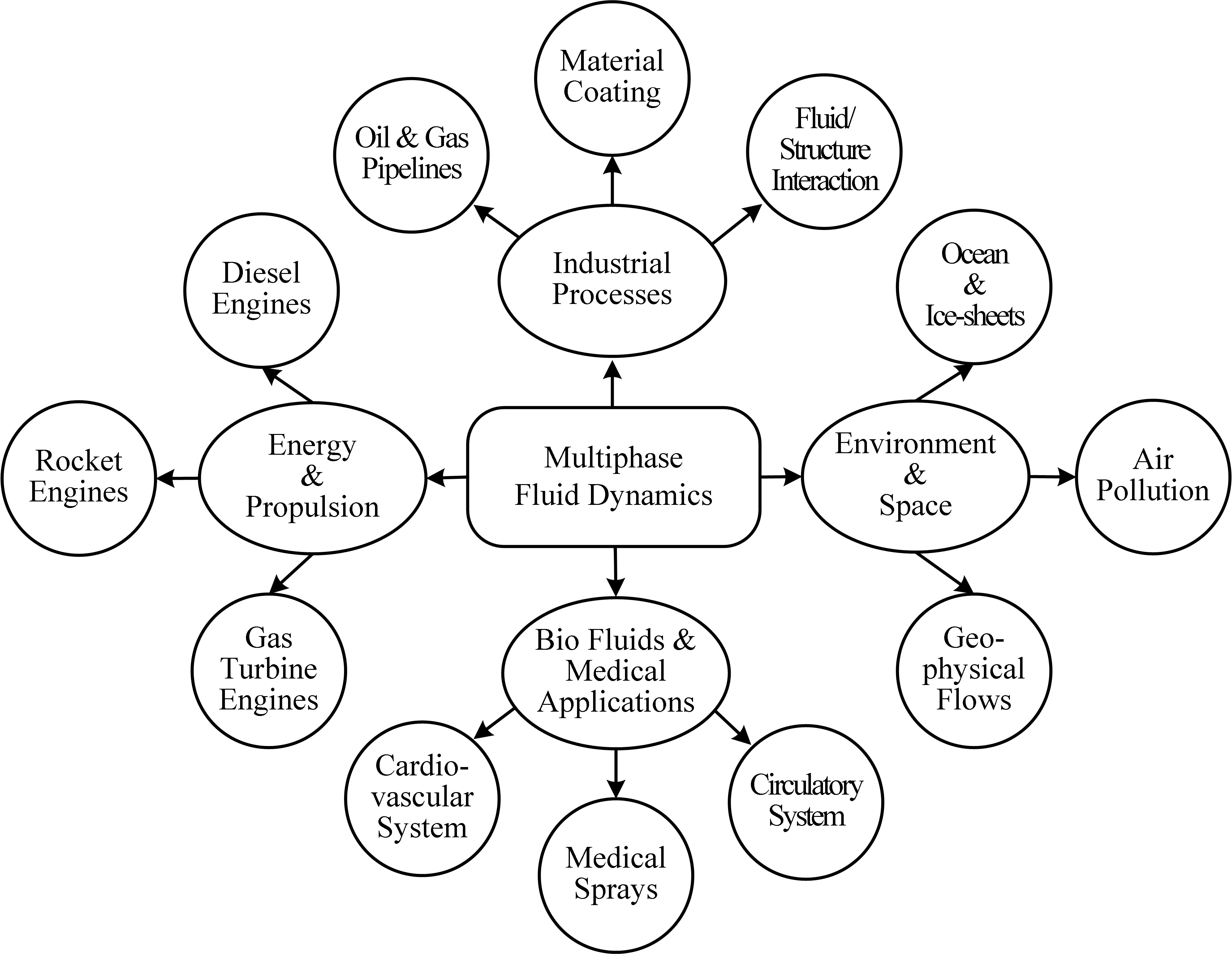
About
About the lab and the PI, click to read more.
Thermal and electrolytic decomposition and ignition of water solutions of hydroxylammonium nitrate (HAN, [NH3OH]+[NO3]-) are investigated. Experiments are conducted to demonstrate the feasibility of electrolytic ignition for HAN-based ionic liquid propellants. As a consequence of electrolysis, the propellant decomposes to NH2OH, HNO3, HONO, HNO, N2O, N2, NO, NO2, H2O, H2(g), and O2(g). These species then initiate a series of gas phase reactions, governed by the H-O-N kinetics scheme, and lead to ignition. Electrochemical mechanisms are established and incorporated into an existing chemical kinetics scheme for thermal decomposition of HAN-water solutions. The analysis treats the liquid and gas phases separately, and matches their respective processes at the interfacial boundary to determine the overall decomposition and ignition characteristics. Results are benchmarked against experimental data for the species evolution in the condensed phase, as well as laser-induced ignition delay of 13M HAN-water solution. The effects of electric current (0-2.5A), propellant volume (0.1-0.3cc), initial temperature (300-450K), and HAN concentration (9-13M) on the ignition behaviors are investigated systematically. The ignition delay decreases with increasing electric current as a power-law function: τign = AI-n, where A = 3.95 and n = 0.95 for a 0.1cc 13M HAN-water solution at an initial condition of 300K and 1atm. The exponent n and coefficient A are decreasing functions of the initial temperature. For a given power, decreasing the initial volume of HAN-water solution increases the current density available for electrolysis, and expedites the ignition process. The work represents the first theoretical study of its kind on this topic.
Relevant Publications
About the lab and the PI, click to read more.

High-fidelity simulations of primary and secondary atomization of liquid fuels. Click here to read more..

Spatiotemporal prediction of non-reacting and reacting multiphase flows using machine learning. Click here to read more.

Decomposition and ignition of liquid fuels. Click here to read more.

Combustion dynamics of reacting gaseous and liquid fuels. Click here to read more.

Read our publications here.

Click here to read more about the high-performance computing software and hardware facilities.

Click here to read about recent events in the group

Main page for the ONR program. Click here to read more.

Click here to locate us on the map and other contact information.